New Paper "Pou5f3, SoxB1, and Nanog remodel chromatin on High Nucleosome Affinity Regions at Zygotic Genome Activation"
Marina Veil, Lev Yampolsky and Daria Onichtchouk found that "Pou5f3, SoxB1, and Nanog remodel chromatin on High Nucleosome Affinity Regions at Zygotic Genome Activation".
Thanks for using Galaxy and congratulation to Marina, Lev and Daria!
Abstract
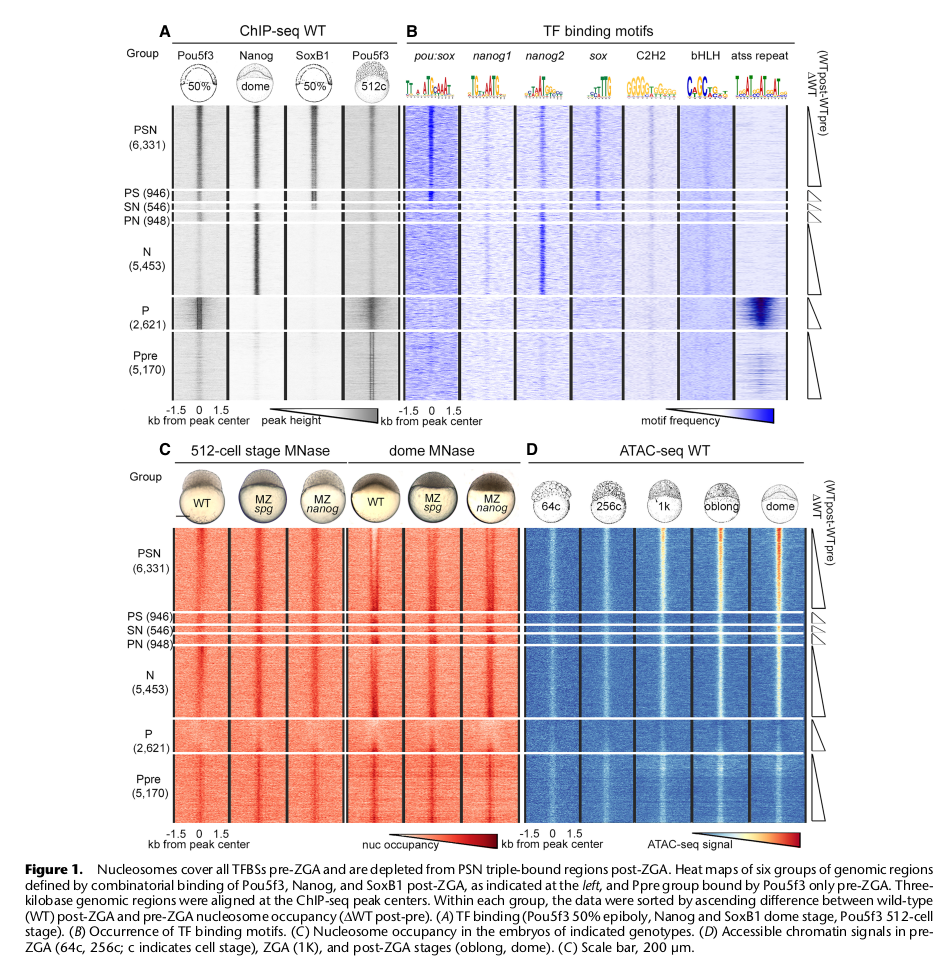
The zebrafish embryo is mostly transcriptionally quiescent during the first 10 cell cycles, until the main wave of Zygotic Genome Activation (ZGA) occurs, accompanied by fast chromatin remodeling. At ZGA, homologs of mammalian stem cell transcription factors (TFs) Pou5f3, Nanog and Sox19b bind to thousands of developmental enhancers to initiate transcription. So far, how these TFs influence chromatin dynamics at ZGA has remained unresolved. To address this question, we analyzed nucleosome positions in wild-type and Maternal-Zygotic (MZ) mutants for pou5f3 and nanog by MNase-seq. We show that Nanog, Sox19b, and Pou5f3 bind to the High Nucleosome Affinity Regions (HNARs). HNARs are spanning over 600 bp, featuring high in vivo and predicted in vitro nucleosome occupancy and high predicted propeller twist DNA shape value. We suggest a two-step nucleosome destabilization-depletion model, where the same intrinsic DNA properties of HNAR promote both high nucleosome occupancy and differential binding of TFs. In the first step, already prior to ZGA, Pou5f3 and Nanog destabilize nucleosomes on HNAR centers genome-wide. In the second step, post-ZGA, Nanog, Pou5f3, and SoxB1 maintain open chromatin state on the subset of HNARs, acting synergistically. Nanog binds to the HNAR center, while the Pou5f3 stabilizes the flanks. The HNAR model will provide a useful tool for genome regulatory studies in the variety of biological systems.